WHAT IS
paraganglioma?
SEE THIS PAGE FOR MORE INFORMATION ON COVID-19 & PHEO PARA
Paraganglioma (păr′ə-găng′glē-ō′mə) is a rare, slow-growing tumor that is closely related to pheochromocytoma. They originate in the parasympathetic or sympathetic nervous system. They occur in both men and women equally, and they affect every race of people. They can occur at any age, but the peak incidence occurs in the third to fifth decades in life.
Like pheochromocytomas, extra-adrenal paragangliomas can produce hormones called catecholamines, which include norepinephrine (noradrenaline), epinephrine (adrenaline), and dopamine. In excess, these hormones can cause persistent or episodic high blood pressure and other symptoms. Some paragangliomas are what doctors refer to as “biochemically silent,” meaning they do not produce any catecholamines. However, they can produce other symptoms like headache, vision problems, hearing loss, dizziness, or hearing your heartbeat (“pulsatile tinnitus”). Extra-adrenal paragangliomas are often found incidentally.
Approximately 85% of paragangliomas are in the abdomen, 12% are found in the chest, and only about 3% are in the head and neck. This figure shows where paraganglioma can be found. Paragangliomas in the head and neck are less likely to be metastatic than tumors in the chest, abdomen and pelvis.
Paragangliomas, if detected early, can be successfully treated and managed in the vast majority of cases. If possible, the treatment of choice for the condition is surgery to remove the tumor(s), but there are other treatment options. Surgical treatment usually alleviates symptoms. Once diagnosed, it is recommended to be seen by a multi-disciplinary medical team with pheo para experience.
30-40% of pheochromocytomas and paragangliomas are hereditary. If you are diagnosed with paraganglioma talk to your doctor about genetic testing. Read more on our genetics page.
Navigate through each section using the tabs on this page or scrolling down.
Watch up-to-date videos on various pheo para topics presented by experts here.
See the National Cancer Institutes Patient Guide to pheochromocytoma.
We asked the same 7 questions to 3 patients, check out their answers here.
Download/print these PPA educational materials.
Download factsheets on Genetic & Sporadic Pheochromocytoma & Paraganglioma on the INCA website.
A patient’s journey from symptoms to diagnosis to treatment, can be a long one. At times, you may feel dismissed by your medical team. You can find clinical practice guidelines here. Sharing this information during appointments can help you communicate your questions and concerns with your medical team.
THE SYMPTOMS
signs to look for
Paraganglioma can occur at any age, but most commonly affects people between the ages of 20 and 50. While very rare, the illness often causes a range of symptoms that when recognized can help with diagnosis. Many of these symptoms can be caused by multiple other conditions as well. Some paragangliomas do not cause any symptoms.
Signs and symptoms may occur weekly, several times daily, or once every few months. Most attacks last less than an hour, but rarely more than several days.
Signs + Symptoms
Triggers of Symptomatic Spells
When to see a Doctor
Signs + Symptoms
Signs or symptoms of paragangliomas may include:
- Hypertension
- Sustained hypertension
- Paroxysmal hypertension
- Orthostatic hypotension
- Headache
- Palpitations+/-tachycardia
- Diaphoresis/sweating
- Anxiety
- Swelling at tumor site
Symptoms from secreting tumors can mimic many other illnesses. Here is a list of differential diagnoses that may be helpful on your journey to a diagnosis.
Less common signs or symptoms may include:
- Pallor
- Flushing
- Hyperglycemia
- Vomiting
- Abdominal/back pain
- Nausea
- Fatigue
- Dyspnea
- Dizziness
- Visual symptoms
Triggers of Symptomatic Spells
Spells may occur spontaneously or may be triggered by such factors as:
- Physical exertion
- Anxiety or stress
- Changes in body position
- Bowel movement
- Labor and delivery
- Surgery and anesthesia
- Caffeine
- Certain drugs such as steroids, decongestants, psychiatric drugs such as phenelzine, tranylcypromine, and isocarboxazid
- Stimulants, such as amphetamines or cocaine
Foods high in tyramine, a substance that affects blood pressure, also can trigger a spell. Tyramine is common in foods that are fermented, aged, pickled, cured, overripe or spoiled. These foods include:
- Some cheeses
- Some beers and wines
- Dried or smoked meats
- Avocados, bananas and fava beans
- Pickled fish
- Sauerkraut or kimchi
Certain medications that can trigger a symptomatic spell include:
- Decongestants
- Monoamine oxidase inhibitors (MAOIs), such as phenelzine (Nardil), tranylcypromine (Parnate) and isocarboxazid (Marplan)
- Stimulants, such as amphetamines or cocaine
Medical procedures/treatments that can trigger a symptomatic spell include:
- Intubation
- Anesthesia
- Endoscopy
- Catheterization
- Chemotherapy
When to see a Doctor
The signs and symptoms of paraganglioma can be caused by a number of different conditions.
- If any of the listed signs or symptoms come and go suddenly, you should see a doctor. It’s important to get a prompt diagnosis.
- Although high blood pressure is a primary sign of a paraganglioma, most people with high blood pressure don’t have a paraganglioma, and not all patients with a paraganglioma have hypertension. Talk to your doctor if any of the following factors are relevant to you:
- Difficulty controlling high blood pressure with current treatment plan
- A family history of paraganglioma
- A family history of a related genetic disorder: multiple endocrine neoplasia, type II (MEN II); von Hippel-Lindau disease; familial paraganglioma or neurofibromatosis 1 (NF1)
Pheo para is often referred to as the “great mimic”. Distinguishing the illness from other conditions can be a challenge. Below are some conditions that may cause similar symptoms.
Endocrine
- adrenal medullary hyperplasia
- hyperthyroidism, thyroid storm
- carcinoid
- hypoglycemia (often due to the presence of insulinoma)
- medullary thyroid carcinoma
- mastocytosis
- menopausal syndrome
Cardiovascular
- heart failure
- arrhythmias
- ischemic heart disease, angina pectoris
- baroreflex failure (syncope, orthostatic hypertension, labile hypernoradrenergic essential hypertension, renovascular disease)
Neurologic
- migraine or cluster headaches
- diencephalic autonomic epilepsy
- meningioma
- POTS (postural orthostatic tachycardia syndrome)
- Guillain-Barre syndrom
- encephalitis
Psychogenic
- anxiety or panic attacks
- factitious use of drugs
- somatization disorder
- hyperventilation
Pharmacologic
- tricyclic antidepressant
- cocaine
- alcohol withdrawal
- drugs stimulating adrenergic receptors
- abrupt clonidine withdrawal
- dopamine antagonists
- monoamine oxidase inhibitors
- ephedrine-containing drugs
- factitious use of various drugs including catecholamines
Miscellaneous
- neuroblastoma, ganglioneuroma, ganglioneuroblastoma
- actute intermittent porphyria
- mastocytosis
- unexplained flushing spells
- recurrent idiopathic anaphylaxis
- lead and mercury poisoning
Adapted from Lenders et al.
DIAGNOSIS
preparing for your appointment
If a para is suspected, you should be referred to a doctor who specializes in hormonal disorders (an endocrinologist).
What you can do
Before your appointment, make a list that includes the following:
- Signs or symptoms — or any changes from normal— that may be causing concern
- A record of the frequency and duration of symptoms
- Recent changes or stresses in your life
- All medications — including over-the- counter drugs and dietary supplements — and doses you take. This is very important because supplements and OTC drugs can affect test results. Always consult your doctor before stopping medications.
- A log of typical food and beverage consumption
- Family history of medical conditions
The twenty-four hour urine and blood (plasma) tests are commonly used if pheochromocytoma or paraganglioma is suspected.
Pheo is often referred to as the “great mimic”. Receiving a diagnosis and distinguishing pheo para from other conditions can be a challenge. In a recent survey, 35% of respondents indicated it took 4+ years to receive a diagnosis.
The 24-hour urine test and the blood plasma test both measure catecholamines and metanephrines (which are metabolites of catecholamines).
Metanephrines are released continuously from the tumor, as opposed to catecholamines, which are usually released intermittently. This continuous release of metanephrines from the tumor explains why testing for metanephrines is preferred over testing for catecholamines. In rare cases, a paraganglioma, often in the head/neck, may not produce catecholamines or metanephrines. In these latter cases, an imaging test is the preferred method for diagnosis.
The 24-hour urine test and the blood plasma test are equally effective at measuring of free metanephrines and ultimately providing a diagnosis. Both tests are approximately 95% accurate. Some laboratories measure only total metanephrines and this is nearly as accurate as the measurements of free metanephrines. Determining which test to use is dependent on your and your doctor’s preference.
Twenty-four-hour urine test:
The 24-hr urine metanephrine test measures the free metanephrines in your urine. A 24-hour urine collection is done by collecting your urine in a special container over a full 24-hour period. This may be less convenient than the blood test. When the 24-hr urine is incompletely collected, the test result may turn out to be false-negative (normal test result in a patient with a pheo). True positive values (abnormal test result in a patient with pheo) are usually two or more times higher than the upper limit of normal.
See the Patient Guide to Urine Metanephrines Testing here.
Blood plasma test:
Plasma free metanephrines test is a blood test that measures the amount of metanephrines in the blood. This test compared to the 24-hour urine test can be more convenient since it is only a one-time blood draw. The Endocrine Society Guideline suggests that blood be drawn after the patient has a brief rest and is supine (lying down on his/her back), but many labs do not follow this guideline. Patients with pheos or paras often have two or more times the upper limit of normal, so retesting in the supine position can be requested if your blood was drawn while you were sitting up and results are indeterminate.
Additionally, blood should be drawn into prechilled tubes, and the blood sample tubes should be put on ice before centrifugation. This is so that the plasma can be frozen until actual measurement. If samples are not drawn into prechilled tubes and put on ice, approximately 20% lower plasma metanephrines may be result.
See the Patient Guide to Blood Plasma Metanephrines Testing here.
3-Methoxytyramine
3-methoxytyramine (3MT), which is the breakdown product of dopamine, is sometimes used for testing. This is a blood test that can be moderately useful in patients with clear symptoms to confirm a pheo para diagnosis. It can also be helpful in identifying patients with head/neck paragangliomas. 3-MT may be measured in patients with genetic mutations, especially SDHB, which has a higher risk of metastatic disease. Finally, it may be useful in the detection of metastatic disease. 3MT testing is however not available in most labs in the U.S.
See more information on 3MT testing here.
False Negative/False Positive/Indeterminate Results
Sometimes test results are false negative (normal test result in a patient with a pheo), a false positive (abnormal test result in patient with no pheo) or indeterminate (levels may be slightly higher than the upper limit of normal). If this latter happens, your doctor may retest you or consider imaging.
Why false negative?
- very small tumors
- tumors that aren’t producing catecholamines
- incomplete urine collections
- blood sample not drawn into pre-chilled tubes or put on ice after sample has been taken
Why false positive?
- blood was drawn in the seated position. The Endocrine Society Guideline suggests that blood be drawn after the patient has a brief rest and is supine (lying down on his/her back), but many labs do not follow this guideline.
- severe pain, heart failure or acute physical or emotional stress.
- supplements, prescribed medications, over-the-counter or illicit drugs, see below for a list that may affect results. This depends however on the technical procedure used to measure.
Tell your doctor about all prescribed and over-the-counter medications, supplements and illicit drugs you are taking. Do not stop taking prescribed medications without consulting your doctor first. Here is a list of medications/substances that may affect test results:
Acetaminophen, sympathomimetics, tricyclic antidepressants, selective serotonin reuptake, serotonin norepinephrine reuptake inhibitors, cyclobenzaprine, levodopa, monoamine oxidase inhibitors, some beta-blockers, some alpha blockers (phenoxybenzamine), marijuana, illicit drugs
Imaging
Imaging, taking pictures of the inside of the body, is often used once biochemical tests indicate a pheo or para. Imaging will help to identify where, how many, and size of the tumor(s). CT/MRI are often used first, before functional imaging is used.
Functional imaging means imaging involving the use of a radioactive substance to take pictures of the body. Some of these radioactive substances can also be used for treatment of metastatic pheo para and this can be confusing. The Society of Nuclear Medicine and Molecular Imaging has many online resources to explain the use of both and address issues about radiation exposure.
CT scan (CAT scan):
A CT scan takes a series of detailed pictures inside the body in places such as the neck, chest, abdomen, and pelvis. The pictures, taken at various angles, are made by a computer linked to an x-ray machine. A dye may be injected into a vein to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
MRI (magnetic resonance imaging):
An MRI uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body such as the neck, chest, abdomen, and pelvis. This procedure is also called nuclear magnetic resonance imaging (NMRI).
Functional imaging:
Functional imaging allows physicians to see how the body is functioning and to measure its chemical and biological processes. For pheo para, functional imaging may include I-123 MIBG, F-18 FDG-PET/CT, F-18 DOPA-PET/CT, Ga-68 DOTATATE PET/CT or Cu-64 (copper) DOTATATE PET/CT scans. A small amount of a radiotracer is injected into the patient’s bloodstream, where it’s distributed throughout the body and accumulates in tissues and organs. As it breaks down, the radiotracer emits photons which are detected by special types of cameras to provide very precise pictures of the area of the body being imaged.
Functional imaging can be used to identify whether a disease has progressed to other parts of the body (metastatic), and where the tumors are located. Because the cost of these scans can be prohibitive, they are not widely available, and they may not be covered by insurance.
Treatment Overview
exploring your options
Newly diagnosed patients can receive a care kit filled with educational and supportive information. See more details below.
Paragangliomas, if detected early, can be successfully treated and managed in the vast majority of cases. If possible, the treatment of choice for the condition is paraganglioma surgery, either open or laparoscopic.
Alternatively, an experienced doctor may suggest only regular monitoring of the tumor(s) if they are not secreting catecholamines, there are no symptoms and the tumor(s) are stable (not growing).
When surgery is not an option due to multiple tumor sites, metastatic disease, or the location of the paraganglioma, chemotherapy, radiation or radiopharmaceutical therapy are also treatment options.
Below details treatment options.
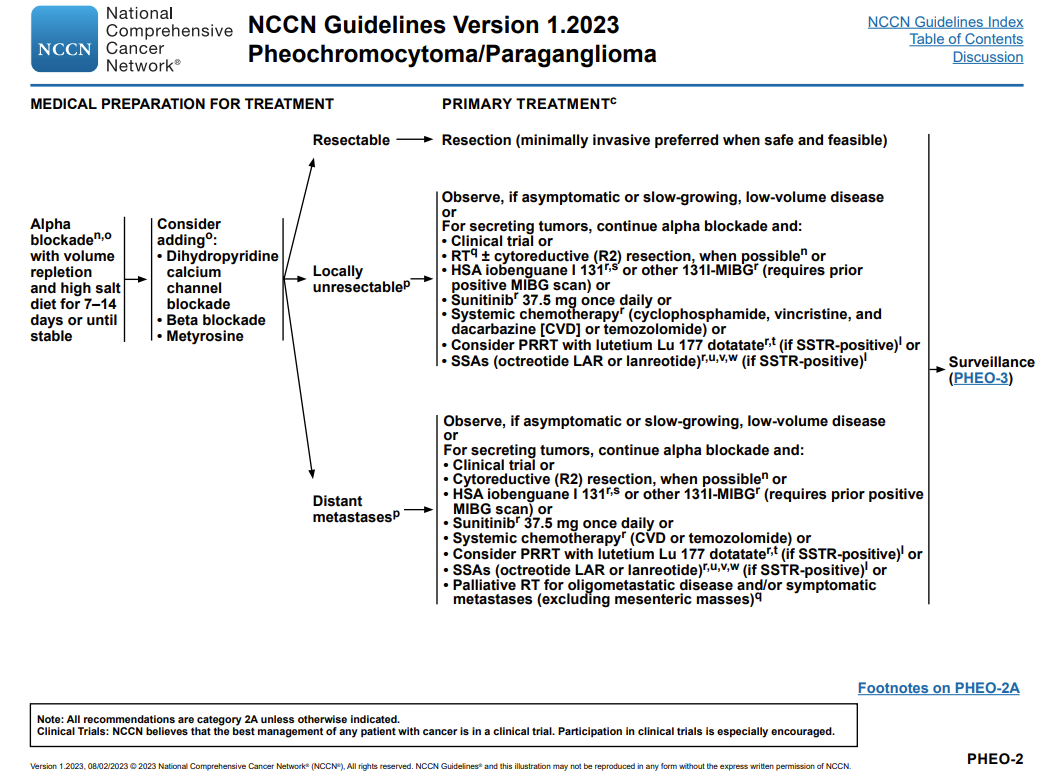
Slide credit: National Comprehensive Cancer Network Guidelines
Belzutifan (Welireg) was approved by the FDA in May 2025 for unresectable and metastatic pheochromocytoma & paraganglioma. See the announcement below and the recorded webinar here to find out more about the results from the clinical trial recently published in October in the New England Journal of Medicine.
Treatment OPTIONS
Surgery
Radiation therapy
Somtaostatin Analogs (SSA's)
Chemotherapy
Ablation therapy
Embolization therapy
Tyrosine kinase inhibitor therapy
Clinical Trials
Surgery
Surgery
Surgery to remove a paraganglioma is the preferred treatment option. During surgery, the tissue and lymph nodes close to the tumor may be checked and if the tumor has spread, these tissues may also be removed. Drugs may be given before, during, and after surgery to keep blood pressure and heart rate normal.
After surgery, the blood or urine are checked to ensure metanephrine levels and subsequently, catecholamine levels have returned to normal. Normal metanephrine levels are a sign that all the paraganglioma cells were removed, if the tumor was secreting catecholamines.
If you have surgery scheduled, you can participate in pheo para research and be a part of finding new treatments or a cure by donating your tumor tissue to research. It is quick and easy to consent. You can learn more here.
Before Surgery
Before surgery the patient must be adequately “blocked” with medication. Going under anesthesia without being blocked is very dangerous. Anesthesia can cause tumors to release massive amounts of catecholamines. Manipulation of the tumor during surgery can also cause this release, which may result in a hypertensive crisis and even death. This is why it is not recommended for patients to have a biopsy of the tumor if pheo or para is suspected.
It is extremely important that practitioners involved in the care of the patient have experience with pheo para surgery and that patients be “blocked” for the best possible outcome. Alpha and beta-blockers are prescribed to normalize blood pressure and heart rate, which protect the patient from the effects of high levels of hormones (catecholamines) released during surgery. First, an alpha blocking medication is prescribed for at least 2 weeks before the surgery. Phenoxybenzamine(Dibenzyline) is commonly prescribed. Doxazosin, prazosin and terazosin are also used. Once blockage has been obtained, a beta-blocker is prescribed, sometimes in combination with calcium channel blockers. A high salt diet may be recommended, as well.
After Surgery: Follow-Up
Urine or plasma tests should be repeated 2-8 weeks after surgery to check for any remaining disease. Long-term regular follow-up is recommended for all patients. Yearly urine or plasma tests for paraganglioma should be performed for life to detect remaining disease, return of the disease, or the development of metastases. For most people, follow-up CT or MRI is not needed if urine and plasma test results are normal. Exceptions to this may include the identification of a genetic mutation or if the primary tumor was large. Read more about genetic mutations on this page.
Radiation therapy
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy:
- External radiation therapy uses a machine outside the body to send radiation toward the cancer.
- Internal radiation therapy or radiopharmaceutical therapy uses a radioactive substance sealed in needles, seeds, wires, or catheters that are placed directly into or near the cancer.
The way radiation therapy is given depends on the type of cancer being treated and whether it is localized, regional, metastatic, or recurrent.
Molecular imaging (using radiation to take pictures of the body) and nuclear medicine (using radiation to treat an illness) can be confusing. The Society of Nuclear Medicine and Molecular Imaging has many online resources to explain the use of both and address issues about radiation exposure.
PRRT Therapy
PRRT (Peptide Receptor Radionuclide Therapy) is a radiopharmaceutical therapy that when injected into the patient’s bloodstream travels to and binds to the tumor delivering a targeted high dose of radiation directly to the cancer cells. A Gallium DOTATE or NETSPOT PET/CT scan is done first to check if the tumor will respond to PRRT.
Lutathera (lutetium Lu 177 dotatate) is used off-label to treat pheo para. Lutathera does not usually require a hospital stay and minimal sterilization precautions are required to limit radiation exposure to those in contact with you.
Some clinical trials are available at the University of Iowa, the NIH, and Cincinnati Children’s Hospital for PRRT. More information can be found at clinicaltrials.gov or by reaching out directly to the institution.
https://incalliance.org/net-info-packs/
MIBG Therapy
Paraganglioma can be treated with MIBG, which is a radiopharmaceutical that is injected into the patient’s bloodstream. It travels to and binds to the tumor delivering a targeted high dose of radiation directly to the cancer cells. Not all paras take up MIBG, so a test is done first to check for this before treatment begins. Approximately 60% of tumors are MIBG active. MIBG Therapy has limited availability.
Somtaostatin Analogs (SSA's)
Somatostatin is a naturally occurring hormone that acts by binding to somatostatin receptor (SSTR), a receptor that is overexpressed in pheo para. SSA’s such as octreotide and lanreotide work by activating SSTR’s, which can slow tumor growth. Studies have produced mixed results on the effectiveness of SSA’s. Octreotide and lanreotide are administered intravenously (by a needle into the body).
Chemotherapy
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). When chemotherapy is placed directly into the cerebrospinal fluid, an organ, or a body cavity such as the abdomen, the drugs mainly affect cancer cells in those areas (regional chemotherapy). Combination chemotherapy is treatment using more than one anticancer drug. The way the chemotherapy is given depends on the type of cancer being treated and whether it is localized, regional, metastatic, or recurrent.
Chemotherapy drugs that may be used can include cyclophosphamide, vincristine, dacarbazine, and temozolomide monotherapy in malignant pheos for SDHB genetic mutations.
Ablation therapy
Ablation Therapy
Ablation is a treatment to remove or destroy a body part or tissue or its function. Ablation therapies used to help kill cancer cells include:
- Radiofrequency ablation: A procedure that uses radio waves to heat and destroy abnormal cells. The radio waves travel through electrodes (small devices that carry electricity). Radiofrequency ablation may be used to treat cancer and other conditions.
- Cryoablation: A procedure in which tissue is frozen to destroy abnormal cells. Liquid nitrogen or liquid carbon dioxide is used to freeze the tissue.
Embolization therapy
Embolization therapy
Embolization therapy is a treatment to block the artery leading to the adrenal gland. Blocking the flow of blood to the adrenal glands helps kill cancer cells growing there.
Tyrosine kinase inhibitor therapy
Tyrosine kinase inhibitor therapy
TKI is a targeted therapy treatment that uses drugs or other substances to identify and attack specific cancer cells without harming normal cells.
Sunitinib and cabozantinib are types of TKI therapy that can be prescribed off-label and are currently being studied in clinical trials.
Clinical Trials
New types of treatment are being tested in clinical trials
Information about clinical trials is available from the NCI website or at clinicaltrials.gov.
Participation in clinical trials for those with progressive disease is critical to finding better treatments for pheo para. It is important to note, that placebos are rarely used in cancer treatment clinical trials. For pheo para, they may be used in a clinical trial that compares standard treatment plus a placebo, with standard treatment plus a new treatment. So, everyone participating in a clinical trial will receive, at least, the standard treatment commonly used for pheo para.
Cancerous Paraganglioma
Rarely, paraganglioma can spread to other organs.
The prognosis for these patients is highly variable and can be based on the location of the tumors, genetic status, among other factors. It is highly encouraged for metastatic patients to receive treatment from an experienced, multi-disciplinary pheo para team. There are currently no cures for cancerous paraganglioma. However, existing treatment options may reduce the tumors and prolong survival.
Metastatic
All paraganglioma have the potential to become metastatic, or spread to other parts of the body. This happens in approximately 15-25% of cases.
It is highly recommended for metastatic patients to receive treatment from an experienced, multi-disciplinary pheo para team. In addition, everyone who has metastatic para should have genetic testing. Knowing your genetic status will help your medical team determine an appropriate course of treatment. Read more on the genetics page.
There are currently no cures for metastatic paraganglioma. However, existing treatment options may reduce the tumors and prolong survival. Some patients live for decades with metastatic paraganglioma. The prognosis is highly variable and is dependent upon the size of the primary tumor (tumors larger than 5-6 cm are more likely to metastasize), levels of methoxytyramine (a metabolite of the neurotransmitter dopamine which can be measured in the blood), and genetic status. Paras that are originally in the head and neck are less likely to metastasize than paras that develop in other areas. Some genetic mutations are more likely to develop metastatic para. You can read more about each genetic mutation and its prognosis on the genetics page. Metastatic para often spreads to the bone and sometimes to the lymph nodes, lungs and liver.
Treatment of metastatic para can include surgery, MIBG therapy, PRRT therapy (Lutathera), chemotherapy, and others. Please see the treatment portion of this page for more information.
Participation in clinical trials for those with progressive disease is critical to finding better treatments for pheo para. Please visit our clinical trials webpage for more information.
Check out this opportunity for a free multi-disciplinary review of your medical records.
Pregnancy
Having a baby can be an exciting and joyous time, but if you or your partner have/had a pheo or para and/or have a genetic mutation, the process can be stressful, and even dangerous, for mom and baby.
The good news is that given special considerations and planning, a healthy pregnancy is possible, even in metastatic patients. The topics below outline some factors to consider when planning to become pregnant or if you have been diagnosed during pregnancy. Regardless of when you receive your diagnosis, all pheo or para patients should have genetic testing. You can find out more information on genetic testing here.

The good news is that given special considerations and planning, a healthy pregnancy is possible, even in metastatic patients. The topics below outline some factors to consider when planning to become pregnant or if you have been diagnosed during pregnancy. Regardless of when you receive your diagnosis, all pheo or para patients should have genetic testing. You can find out more information on genetic testing here.
The importance of an experienced multi-disciplinary team is especially critical in the case of pregnant patients. A coordinated team with various specialists must address topics such as when during pregnancy you are diagnosed, degree of catecholamine excess, location of tumor(s), metastatic or localized, and genetic predisposition. To find an experienced team, check out our Center of Excellence program here.
You can share these clinical best practices with your medical team – https://pubmed.ncbi.nlm.nih.gov/31345526/
https://pubmed.ncbi.nlm.nih.gov/33961120/
Watch this webinar on pheo para and pregnancy and see the Q&A portion with questions submitted by patients.
Family Planning
If you or your partner have a genetic form of pheo para, your child will have a 50/50 chance of inheriting the genetic mutation. Read more about genetic mutations here.
In this case, pre-implantation genetic diagnostic (PGD) testing can be used with in vitro fertilization (IVF). PGD tests the embryos prior to implantation to only implant embryos that do not have the genetic change seen in you or your partner.
If you are already pregnant, and prenatal genetic testing is desired, consultation with a prenatal genetic counselor (findageneticcounselor.com) is recommended. There are genetic testing options using chorionic villus sampling (CVS) and/or amniocentesis at various points in the pregnancy that can be performed, and a full consultation should occur with a specialist.
For those planning to become pregnant there is no evidence that suggests fertility treatments, estrogen, progesterone or pregnancy can trigger the formation or growth of a tumor, or that previous tumors affect fertility.
Diagnosis Before Pregnancy
If you have had or currently have pheo para, or if you have the metastatic form of the illness, planning is critical to ensure the best possible outcomes for mother and baby. This may include planning for imaging and diagnostic testing, taking alpha and beta blockers and calcium channel blockers, method of delivery and more. For example, biochemical testing, cross-sectional imaging (MRI/PET/CT), and functional imaging may be considered before conception. Because of the risk of radiation exposure, functional imaging is not recommended during pregnancy. You can learn more about functional imaging here under the diagnosis section.
Diagnosis During Pregnancy
The occurrence of a diagnosis during pregnancy is estimated at 1 in 15,000 to 54,000 pregnancies. Diagnosis for non-pregnant individuals is often difficult, this may be even more difficult during pregnancy because symptoms of both preeclampsia and pheo para may appear identical to clinicians, but there are some differences.
Hypertension as a result of pheo para can develop throughout pregnancy, whereas preclampsia usually appears in the last 20 weeks of pregnancy. Headaches and abdominal pain are associated with both preeclampsia and pheo para. Pheo or para-related hypertension is generally not associated with ankle swelling, proteinuria, or an increased plasma uric acid level. Finally, orthostatic hypotension, which happens when standing up from sitting or lying down, can be a symptom for pheo para, but not preclampsia.
| Symptoms | Pheo or Para
(symptoms occur throughout pregnancy and may worsen) |
Preclampsia
(symptoms during the last 20 weeks of pregnancy) |
| Hypertension | X | X |
| Orthostatic Hypotension | X | |
| Headache/Abdominal Pain | X | X |
| Ankle Swelling, Proteinuria, Increased Plasma Uric Acid | X |
The method of diagnosing pheo or para is the same as in non-pregnant individuals, using 24-hour urine or plasma metanephrine testing. You can find more information about diagnosis under the diagnosis section of this page. Unfortunately, pheo para symptoms may worsen throughout pregnancy.
For those diagnosed during pregnancy, your team will likely prescribe alpha (phenoxybenzamine
and doxazosine) and then, later on, beta blockers. Both of these will protect you and the baby from catecholamine excess. Calcium channel blockers may also be prescribed if high-blood pressure is still not well controlled. Balancing the efficacy of these medications with potential side effects takes some time, but it is critical to ensure a healthy mom and baby.
If your symptoms are well-controlled with medication your team may suggest delaying surgery until after delivery. If surgery is recommended it is usually optimal before 24 weeks of gestation. After surgery, symptoms should subside, but this can take time.
Delivery
For patients who are postponing surgery to remove the tumor(s) until after delivery, there are many factors to consider when planning for delivery such as the number of previous pregnancies, previous delivery method, whether symptoms are well-controlled with medication, and your personal preference. Many specialists will be involved. Epidural, general, or combined anesthetic techniques have been used successfully for cesarean delivery. In most cases, cesarean is the preferred delivery method.
Post-Pregnancy
Congratulations on your new delivery! New moms are often prescribed medication. It is important to keep in mind that different kinds of frequently used drugs including metoclopramide, steroids, and sympathomimetics can cause symptoms that result in a pheo para crisis[1].
If your medical team suggested waiting until after you give birth to surgically remove your tumor(s), it will most likely be scheduled after you recover from delivery. After the tumor(s) are surgically removed, symptoms should subside, but this can take time. Also, remember that you must be monitored, most likely for the rest of your life, to catch new tumors early.
[1] Eisenhofer, G., Rivers, G., Rosas, A.L. et al. Adverse Drug Reactions in Patients with Phaeochromocytoma. Drug-Safety 30, 1031–1062 (2007). https://doi.org/10.2165/00002018-200730110-00004
