Written by Paula Długosz, PhD, Caregiver
Research Rookies is an ongoing article series designed to help patients and caregivers understand the research that is being done to find answers to questions about pheo para.
Peptide receptor chemoradionuclide therapy for NET patients. The following information is not specific for pheochromocytoma and paraganglioma, but may be investigated by researchers in the future for pheo and para.
In the last Research Rookie article “Outsmarting our pheos and paras! Molecular Radioactive Trojan Horses are Being Used to Beat Pheochromocytoma and Paraganglioma”, we described details of Peptide Receptor Radionuclide Therapy (PRRT). PRRT has been shown to be effective in a vast group of patients whose tumors are somatostatin receptor (SSTR) positive. However, some patients with somatostatin receptor (SSTR) positive tumors do not get better after PRRT and need other therapeutical approaches. Doctors and scientists are constantly looking for new, more effective treatment options. One of these treatments could be so-called peptide receptor chemoradionuclide therapy (PRCRT), which combines PRRT and chemotherapy. However, in PRCRT, chemotherapy is not given at its full therapeutic dose; the chemotherapy is primarily used as a radiosensitizing agent to enhance an effect of PRRT, rather than a direct treatment in itself. Please note that following our summary of PPRCT below, we have provided a glossary of bold terms that may be unfamiliar to you.
We have known for a long time that cells have special repair mechanisms that allow them to sometimes survive after radiation is applied. Addition of chemotherapy to radiation, called chemoradiation (CRT), can sensitize tumor cells and decrease their ability to repair after radiation. This effect leads to enhanced therapeutic efficacy compared to radiation alone.
The first combination therapy of PRRT (177Lu-DOTATATE) and chemotherapy (capecitabine) in 7 patients with gastroenteropancreatic neuroendocrine tumors (GEP-NETs) was conducted in Erasmus MC, Rotterdam, Netherlands. This combination treatment was shown to be feasible, with manageable toxicities. Efficacy of the treatment was not a subject of this study; however, tumor size reduction was seen in some patients.
Based on the experience in Rotterdam, the team at Peter MacCallum Cancer Centre, Melbourne, Australia, led by Prof. Rodney J. Hicks, started treating NET patients in 2006 with combination of PRRT and radiosensitizing chemotherapy (177Lu-DOTATATE and 5-fluorouracil). A high proportion of patients with progressive NET or uncontrolled symptoms received therapeutic benefit from this combination therapy. One year later, a retrospective study from the same medical centre presented favourable outcomes of the combination therapy PRCRT in patients with fluorodeoxyglucose (18F-FDG)-positive neuroendocrine tumours. Fifty-two patients selected for treatment with PRCRT (177Lu-DOTATATE and 5-fluorouracil) had tumours positive for somatostatin-receptor (SSTR) and for 18F-FDG. Patients chosen for combination therapy struggled with progressive disease or had uncontrolled symptoms on conventional therapy. Median progression free survival (PFS) was 48 months, which was significantly superior to other studies with other treatments. Anatomical response accordingly to RECIST could be evaluated in 40 patients at 3 months after completion of PRCRT. 1 patient (2%) showed a complete response (CR), 11 patients (28%) a partial response, 27 patients (68%) stable disease, and only 1 patient (2%) progressed on PRCRT.
But what is 18F-FDG PET/CT imaging and which group of patients usually have FDG-positive NET tumors?
18F-FDG PET/CT scan uses a so-called fluorodeoxyglucose (FDG) in which a radioactive atom (18F) is applied to a glucose molecule. 18F-FDG is administered to a patient before the 18F-FDG PET/CT scan. Doctors can observe which organs/cells have a high uptake of glucose with radioactive atoms. Some healthy cells in your body (e.g., cells in the central nervous system and immune cells) have high levels of glucose metabolism and would appear positive on 18F-FDG PET/CT scan. Additionally, fast multiplying cancerous cells (called high-grade tumors) will also accumulate a significant amount of 18F-FDG because of their high glucose metabolism rate. Therefore, they also appear positive on the 18F-FDG PET/CT scan.
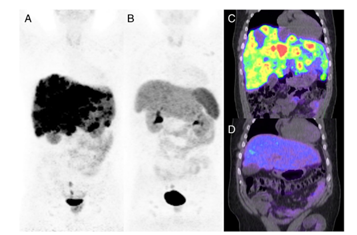
Figure 2 A 46-year-old man with metastatic rectal neuroendocrine carcinoma (ENETS grade 3, Ki-67 40 %) who progressed on chemotherapy and long-acting octreotide hormonal therapy was treated with four cycles of PRCRT. 68Ga-DOTATATE PET/CT before therapy (a, c) demonstrates high somatostatin receptor expression in widespread diffuse hepatic metastases. 68Ga-DOTATATE PET/CT after PRCRT (b, d) presents near complete imaging response. Figure adapted from Kashyap et al., 2014
From a few other studies about PRCRT in NETs we can learn following:
- In a retrospective study, Ballal et al. have shown that a combination of 177Lu-DOTATATE and capecitabine was highly effective in delaying time to progression and improving overall survival in metastatic NETs, when compared to 177Lu-DOTATATE alone.
- A Clinical trial phase I/II study to evaluate efficacy and safety of 177Lu-DOTATATE and CAPTEM (capecitabine and temozolomide) combination was performed in advanced low-grade NETs. Combination of these two drugs resulted in tumour control and stabilization of disease in 94% of 33 patients. Median progression free survival (PFS) reached 31 months.
- The same group of doctors also conducted a phase II clinical study on patients with advanced progressive grade 1 or 2 pancreatic NETs with combination of 177Lu-DOTATATE and chemotherapy. None of the patients had disease progression; partial response was observed in 70% and complete response in 13% of patients. Median progression-free survival was 48 months.
- From a study conducted by Yordanova and colleagues (medical clinics in Mainz und Bonn, Germany), we can learn that after combination therapy (PRRT and CAPTEM or PRRT and temozolomide) clinical benefit (objective response and stable disease) was achieved in 55% of patients according to RECIST criteria. In total, 15 patients suffering from somatostatin-positive, G2/G3 grade NETs (mainly of pancreatic origin) were treated with combination therapy. Median progression-free survival was 7.1 months. Tumor control ratio was also much lower than in studies performed in Melbourne, Australia (55% vs 94%). The authors of the German study suggested that shorter progression free survival and lower tumor control ratio could be caused by extensive pre-treatment of patients participating in this study.
- Kong and colleagues have shown that bulky tumors, including those with higher ENETS grade (Grade 2 and 3), can be treated with PRCRT starting with 1-2 cycles of 90Yttrium-DOTATATE followed by 2-3 cycles of 177Lu-DOTATATE cycles.
- In studies described above, chemotherapy was not given at its full therapeutic dose. To enhance effect of radiation from PRRT, chemotherapy was used in lower doses as a radio-sensitizer. Doctors from Mumbai Centre tried a different approach in which patients received a full chemotherapeutic dose of CAPTEM in between 2 cycles of 177Lu-DOTATATE peptide receptor radionuclide therapy (PRRT). They called it sandwich chemo-PRRT (SCPRRT). Most toxicities following SCPRRT were low grade (nausea and vomiting, anemia, thrombocytopenia, and leukopenia of grade 1/2). There was a low incidence of grade 3 toxicity and there was no grade 4 toxicities observed. Despite unfavorable factors (high tumor burden, FDG-positive disease and progressive disease), partial response (PR) was found in 17 patients (45%), stable disease (SD) in 15 patients (39%), and progressive disease (PD) in 6 patients (16%). There was a disease control rate (DCR) of 84% following sandwich chemo-PRRT based upon RECIST criteria. Additionally, sandwich chemo-PRRT resulted in a long progression free survival.
Conclusion remarks and perspective for PPGL patients
Several studies (mostly retrospective) have shown favorable response rates and acceptable toxicity profile of “sandwich chemo-PRRT” in a subset of NET patients with aggressive (i.e., grade 3), both SSTR and FDG-avid, metastatic progressive disease. Two main differences should be considered in the setting of PPGL.
- Unlike other NET, high FDG uptake observed in PPGL is most often related to their underlying genetic background rather than their pathologically aggressiveness. It has been shown that PPGL related to mutations in one of the SDHx genes usually exhibited high FDG avidity, regardless of their proliferation index assessed by Ki-67 immunostaining score on pathological specimens. Therefore, high Ki-67 index would probably be a more reliable marker that FDG uptake in the selection of SDHx-related metastatic PPGL for potential chemo-PRRT. For sporadic metastatic PPGL that usually have low-to-moderate FDG uptake, FDG-PET study would probably be more informative in addition to Ki-67 score but this would require further studies.
- Despite their potential aggressive behaviour, most metastatic PPGL display slower proliferation rates than to their high grade GEP-NET counterparts. This most “indolent” clinical behaviour is often associated with higher DNA repair capacity and increased radioresistance. Therefore, beyond association of PRRT with sensitizing or alkyling agents, an important focus should be directed towards synergistic drugs that inhibit DNA repair (e.g., PARP inhibitors, bortezomib-type proteasome inhibitors). However, a thorough understanding of all associated toxicities is imperative to ensure that patients can achieve maximal clinical benefit.
Glossary:
18F-FDG PET/CT scan uses a so-called fluorodeoxyglucose (FDG) in which a radioactive atom (18F) is applied to a glucose molecule. The scan is performed to detect organs/cells that have a high glucose metabolism rate, which includes cancerous cells in your body.
Anatomical response – reduction in tumor size
Anemia – decreased levels of red blood cells circulating in your body. Anemia is diagnosed when levels of hemoglobin, protein that carries oxygen and is present in all red blood cells, is less than 13.5 g/dL in a man or less than 12 g/dL in a woman.
to differentiate – is a process of cell specialization to gain particular characteristics that are important to perform a defined function in your body
Leukopenia- decreased levels of cells responsible for fighting pathogens in our body; these cells are called white blood cells (leukocytes). An adult person with leukopenia has fewer than 3,500 white blood cells per microliter of blood. Decreased levels of leukocytes lead to higher risk of infections.
progression free survival (PFS) – is an amount of time during which a patient’s disease did not get worse (i.e., there was no growth of existing tumors and no presentation of new metastases) after a newly studied drug was used to treat a patient. PFS is used in clinical trials to determine how well a studied drug works. The most effective therapeutics are characterized by a long progression free survival.
RECIST – abbreviated from Response evaluation criteria in solid tumors – is a set rules which help to evaluate how well a patient responds to a treatment. The criteria are based on imaging techniques (x-rays, CT scans, or MRI scans). The types of responses that patient can have after a treatment are: complete response (CR, disappearance of all tumors), partial response (PR, at least a 30% shrinkage in tumors size), stable disease (SD, neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for PD) and progression disease (PD, at least a 20% increase in tumors size or appearance of one or more of new tumors).
Thrombocytopenia – decreased levels of platelets (also called thrombocytes, which are cells in your body responsible for blood clotting and faster wound healing. Thrombocytopenia occurs when platelets levels are lower than 150,000 cells per microliter of blood.