Research Rookies is an ongoing article series designed to help patients and caregivers understand the research that is being done to find answers to questions about pheo para. It’s written in easy-to-understand language focused on making us all better patient and caregiver advocates and providing much-needed hope to those affected.
Written by Paula Długosz, PhD, Caregiver
Outsmarting our pheos and paras!
Molecular Radioactive Trojan Horses are Being Used to Beat Pheochromocytoma and Paraganglioma
Unfortunately, many of us know how limitations in treatment options for unresectable and malignant pheochromocytoma or paraganglioma can hamper management of these rare diseases. In 2018, we welcomed two important breakthroughs in the treatment of pheochromocytoma and paraganglioma. Both of them use systemic radiation therapy to deliver high doses of radiation to the cancerous cells but little to the healthy, surrounding cells.
In July 2018, the U.S. Food and Drug Administration (FDA) approved high-specific-activity I-131 meta-iodobenzylguanidine (HSA I-131 MIBG, brand name: AZEDRA®, Progenics Pharmaceuticals, Inc.) for treatment of adults and adolescents ages 12 and older with unresectable, advanced localized or metastatic pheochromocytoma or paraganglioma that are norepinephrine transporter positive. You can find more information about Azedra here.
In the same year, the FDA also approved lutetium Lu 177 DOTA-TATE (brand name: Lutathera®, Advanced Accelerator Applications S.A.) for treatment of a somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors (GEP-NETs).
For patients with pheochromocytoma or paraganglioma (PPGL) Lutathera® must be prescribed “off-label”. However, multiple clinical trials in PPGL are ongoing. Results of a retrospective study which involved 15 patients of metastatic or unresectable PPGL treated with Lutathera® were recently published.
Although, there is currently no cure for metastatic pheochromocytoma these treatment options can control the growth of tumors, reduce symptoms and prolong survival, which is good news for patients who previously had very few options.
What do Lutathera and Azedra have in common and how do they work?
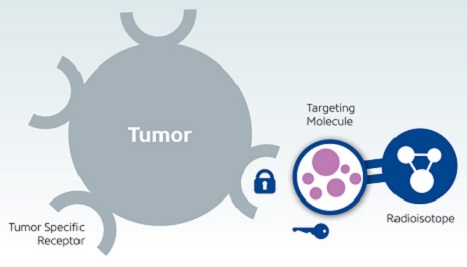
Both treatments belong to a group of targeted radiopharmaceuticals that are used to either image or treat a disease. These treatments are composed of a radioactive compound (radioisotope) linked to a specific molecule, often called a ligand. A specific ligand fits to a specific receptor/transporter on the cell surface like a key fits to a lock. When a circulating ligand finds a fitting receptor/transporter on a particular cell, it binds to the cell and is allowed to enter. Inside the cell, a radioisotope linked to the ligand releases high energy radiation, which damages cellular structures, causes DNA breaks, and, in the end, leads to cell death. Thus, the targeted radiopharmaceutical acts like a Trojan horse by tricking the pheo para cell into thinking it is something that the cell needs, but actually the radioisotope is carrying something that will destroy it!
How do these radioactive trojan horses target mostly cancerous cells and do not enter all cells in your body? Without this specific targeting, healthy cells would be harmed. Researchers noticed that neuroendocrine tumors display a higher density of somatostatin receptors and/or norepinephrine transporters on their cell surfaces than do normal cells. This discovery accelerated the development of molecules with a very similar structure (analogues) to somatostatin and norepinephrine. These analogues serve as ligands for somatostatin receptors and norepinephrine transporters, which means they would be mostly “taken up” by the neuroendocrine tumours and not the healthy cells. Linking these analogues to radioisotopes generated the radiopharmaceuticals called Lutathera® and Azedra®.
Source: cancer.gov
In case of Azedra®, Iobenguane (also called metaiodobenzylguanidine, MIBG), an analogue of norepinephrine, is linked to the iodine-131(131I) radioisotope. Before starting a treatment with I-131-MIBG, one should consider that it comes in two different variants. Low specific activity (LSA)-I-131-MIBG contains a considerable amount of nonradioactive (unlabelled) MIBG. High specific activity (HSA)-I-131-MIBG (commercial name: Azedra®) contains little or no unlabelled MIBG, which results in much higher efficacy and safety.
In case of Lutathera®, (lutetium Lu 177 DOTA-TATE), a chemical linker tetraxetan (DOTA) connects a somatostatin analogue called Tyr3-octreotate (TATE) with lutetium-177 (177Lu) radioisotope. Radiation released from 177Lu only penetrates a short distance in a tissue (<2mm), which indicates that it works better for smaller tumors and minimizes toxicity for surrounding healthy cells. Another type of radioisotope linked to DOTA-TATE is ytrium-90 (90Y), which has a larger radiation range and may be more suitable for larger tumors.
Lutathera® and Azedra® therapies represent examples of precision medicine. Only patients having tumors with an abundance of somatostatin receptors or norepinephrine transporter can qualify for these treatments. The amount of somatostatin receptors and norepinephrine transporters displayed on a tumor(s) cells varies considerably across pheo and para patients or even across multiple tumors that may be present in one patient. For these reasons, an initial scan, prior to therapy, is performed to assess the amount of radiopharmaceutical binding to target receptors on tumor cells.
Gallium-68 DOTA-TATE is used to image somatostatin-rich tumors and iodine-123 MIBG to detect norepinephrine-rich tumors. Gallium-68 radioisotope is a positron emitter and can be imaged using a positron emission tomography (PET) scanner. Iodine-123 gives off gamma rays and can be imaged by a gamma camera (scintillation camera) during a gamma scan (scintigraphy). The above-mentioned scans also have to be regularly performed during therapy to ensure that the therapy is effective.
Unfortunately, radiopharmaceuticals can cause multiple side effects. The major ones are generalized fatigue, nausea and vomiting, renal and hepatic toxicity, and also hematologic (blood) toxicities leading to low blood counts. Consideration of potential side effects should always be addressed.
(Special thanks to our Medical Advisory Board for reviewing this article.)
More info:
To enhance efficacy of PRRT in neuroendocrine tumors, a few combination options are also being studied and considered:
PRRT Combined with Chemotherapy in Patients with Neuroendocrine Tumors
Alternating 90Y and 177Lu cycles
PRRT combined with 131I MIBG
Learn more about participating in PRRT or MIBG clinical trials below:
PRRT:
https://clinicaltrials.gov/ct2/show/NCT03206060
https://clinicaltrials.gov/ct2/show/NCT04614766
https://clinicaltrials.gov/ct2/show/NCT04276597
https://clinicaltrials.gov/ct2/show/NCT04711135
PRRT, Europe:
https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-002951-39/SE
https://www.clinicaltrialsregister.eu/ctr-search/trial/2009-012260-14/SE
MIBG:
https://clinicaltrials.gov/ct2/show/NCT01838187
https://clinicaltrials.gov/ct2/show/NCT01590680
https://clinicaltrials.gov/ct2/show/NCT00107289
https://clinicaltrials.gov/ct2/show/NCT01850888
https://clinicaltrials.gov/ct2/show/NCT03015844
https://clinicaltrials.gov/ct2/show/NCT03649438
https://clinicaltrials.gov/ct2/show/NCT01163383
Learn more about the radiotherapies below:
http://www.snmmi.org/Patients/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6789871/
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6678905/pdf/cancers-11-01018.pdf
https://www.karger.com/Article/Fulltext/499497
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6301943/
https://www.ajronline.org/doi/full/10.2214/AJR.18.19953
https://clincancerres.aacrjournals.org/content/early/2021/03/08/1078-0432.CCR-20-3703
https://clincancerres.aacrjournals.org/content/27/11/2989.full-text.pdf

